NB (Neonatal Bilirubin) testing is crucial in testing paediatric patients for Bilirubin levels in conditions associated with neonatal jaundice, which can have serious consequences if left untreated. Therefore, the importance of using independent quality controls to analyse QC regularly has never been more critical in giving laboratories confidence in releasing patient results.
Multichem® NB (Neonatal Bilirubin) Control is intended for use as a third party, liquid stable quality control material to monitor the precision of laboratory testing procedures for Bilirubin, Theophylline and Caffeine Assays.
Click here to contact us to find out more.
Neonatal Care
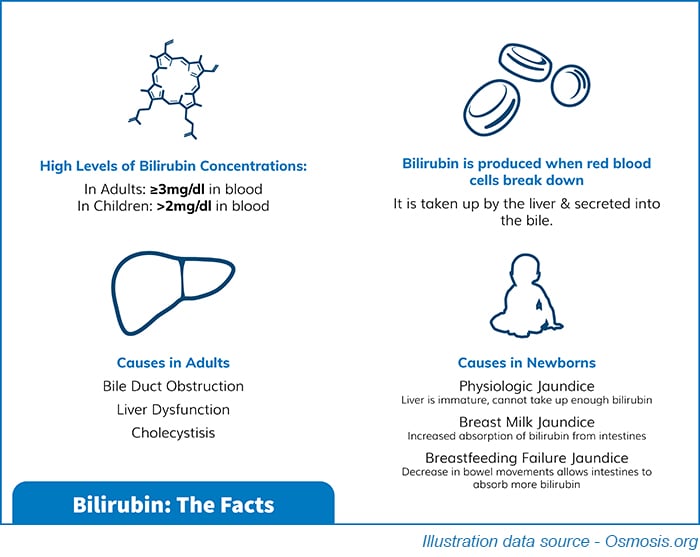
Analysing bilirubin levels is crucial in neonatal care as high levels can lead to jaundice, which if left untreated, can have serious consequences. Bilirubin is a waste product created when red blood cells break down, and neonates have difficulty eliminating it from their bodies. Therefore, accurate measurements are essential to monitor bilirubin levels and prevent complications.
In addition to bilirubin, Multichem® NB includes theophylline, a therapeutic drug that is given to Neonates to improve lung capacity, where it would be required to monitor for toxicity risk. Caffeine is also a listed analyte as this has a clinical function as the primary treatment of the breathing disorders apnea of prematurity.
Multichem® NB is also intended as a third party control to monitor the precision of laboratory testing for liver failure patients, where bilirubin is a crucial biomarker, along with Theophylline and Caffeine analytes.

Discover why it's important for laboratories testing neonatal jaundice in pediatric patient samples to analyze QC regularly, using independent quality controls.
Benefits
Satisfies regulatory guidelines for use of independent controls
Features
Frozen Liquid stable,
single level control
Optimised and assayed for major IVD platforms
Concentrations targeted at clinically relevant levels
14 days open vial stability once thawed and stored at
2 to 8 degrees
Human based materials used as part of manufacturing process
Long shelf life extends lot availability
36 months shelf life once stored at -20 to -80°C degrees
Mimics performance of commutable patient samples
Track product performance with IAMQC
Third party independent quality control
